タグ: AAV
-
気になる企業 – BioReliance/Carlsbad – CDMO of AAV gene therapy [2020/06/18]
Post Views: 476 BioReliance Viral and gene therapy manu…
投稿者

-
気になる企業 – LONZA – 遺伝子治療薬の製造も含めたバイオロジクスCDMOとして投資を進め世界の需要に応える [2021/05/29]
Post Views: 901 LONZA 動向 Lonzaの投資状況から、世界の需要は更に膨らんでいることが…
投稿者

-
[Bio-Edu] ウイルスベクターの比較 – AAV – Adenovirus – Lentivirus – by Vigene Biosciences社 [2020/6/15]
Post Views: 871 Vigene Biosciences Vigene Biosciencesは、…
投稿者

-
気になる企業 – WuXi Advanced Therapies – 遺伝子治療CDMO [2020/06/15]
WuXiグループは、抗体医薬に続き遺伝子治療においても、バイオロジクスのCDMOとして投資に余念がない
投稿者

-
気になる企業 – Charles River – ID14016 [2021/01/21]
Post Views: 901 Charles River チャールスリバーはCROです.医薬品の研究開発のあ…
投稿者

-
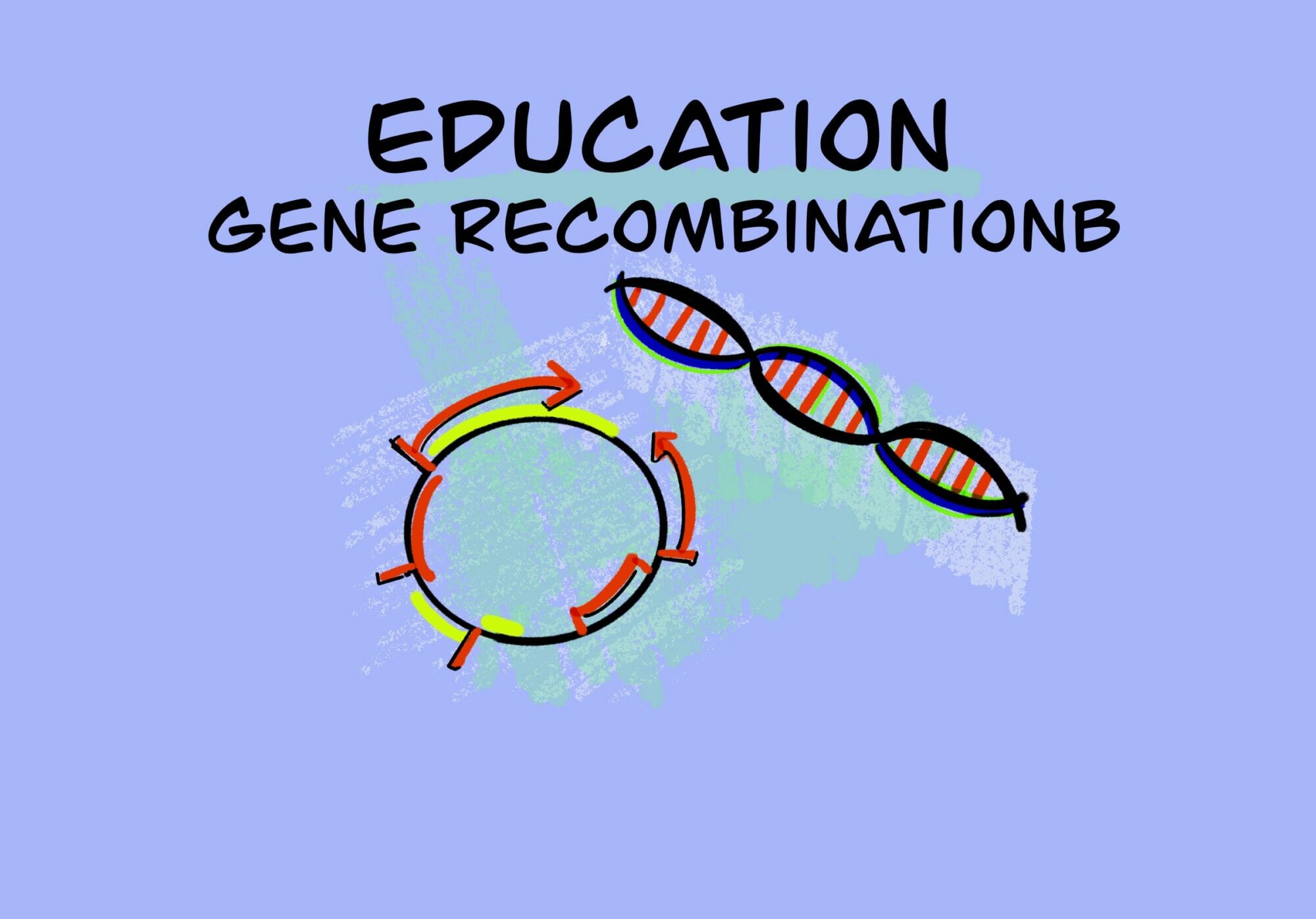
[rAAV] 特許 0010468 – rAAV vector精製 – アパタイト・クロマト精製法 (レジメ)- ID13457
Post Views: 750 METHODS FOR PURIFICATION OF RECOMBINANT…
投稿者

-
[rAAV-Design] – 治療用AAV Vectorの設計 – 考慮事項 – ID12947 [2020/06/24]
Post Views: 837 もとの設計図 天然のアデノ随伴ウイルス(AAV)のゲノムは、1本鎖DNA (s…
投稿者

-
気になる企業 – BioMarin – 世界の開発競争を尻目に、難しいとされいた血友病Aに対する遺伝治療薬をAAV5で開発 – BLAリジェクト、P3追跡データ2年を求められる – [2020/09/02]
Post Views: 815 ID12872 BioMarin 血友病Aに対する遺伝子治療薬が現実なもにとし…
投稿者

-
気になる企業 – VIROVEK (バイロベック) – AAV-toxin Vector技術 – ID12859 [2020/03/31]
Post Views: 759 ID12859 VIROVEK VirovekはSf9/vaculovirus…
投稿者

-
[rAAV-Production] – 治療用AAV Vector製造 – 考慮事項 – SM-ID12844 [2020/10/14]
Post Views: 936 ウイルス・ベクターと宿主細胞の準備 目標のAAVベタクー発現量 15cm ディ…
投稿者