タグ: Bioreliance
-
気になる企業 – BioReliance/Carlsbad – CDMO of AAV gene therapy [2020/06/18]
Post Views: 476 BioReliance Viral and gene therapy manu…
投稿者

-
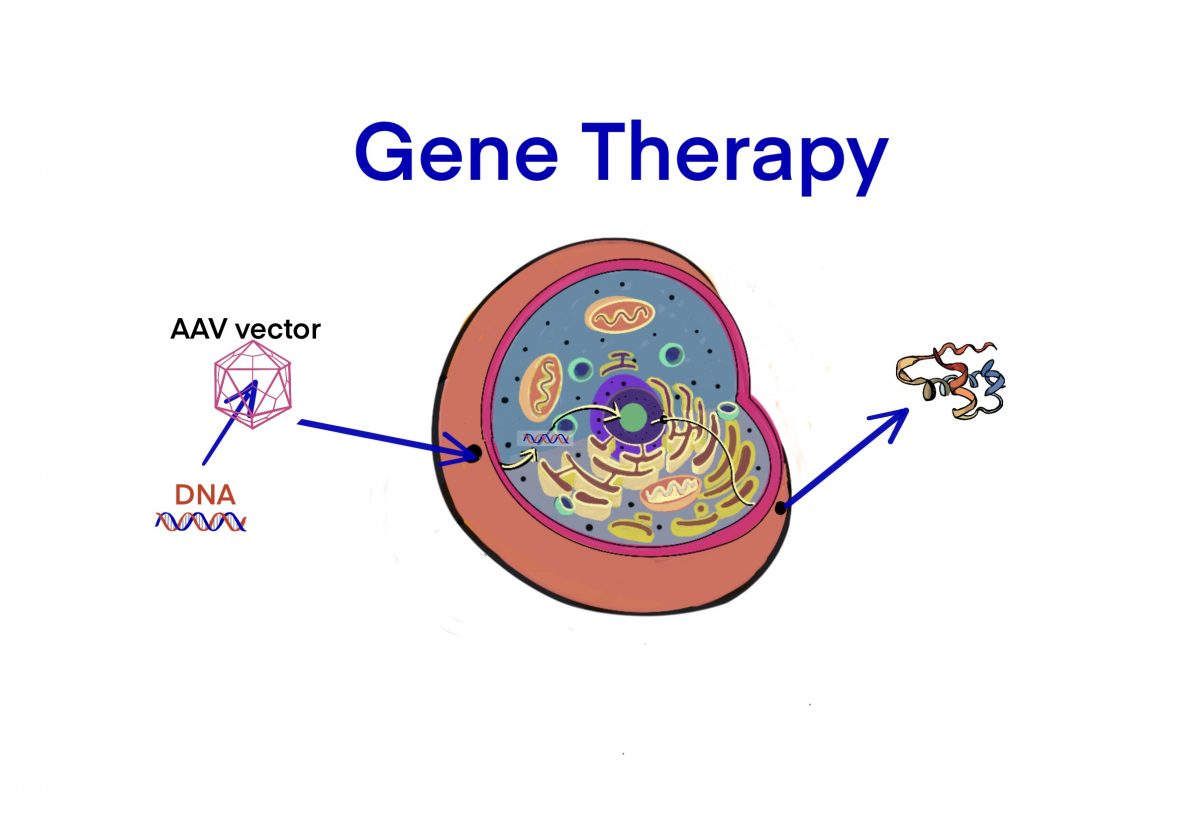
[Bio-rAAV] 遺伝⼦治療における代表的な委託製造会社 (CMO) – ID1881 [2021/02/08]
Post Views: 469 rAAV vectorを製造するCMOリスト 企業サイト サイト 製造サイト …
投稿者